Glaucoma
Glaucoma is a group of eye disorders characterized by progressive damage to the optic nerve, which can lead to irreversible vision loss and, ultimately, blindness if left untreated. It is one of the leading causes of blindness worldwide, and due to its typically asymptomatic nature in the early stages, it is often referred to as the “silent thief of sight.” Early detection and treatment are crucial in preventing significant visual impairment.
The disease is most commonly associated with elevated intraocular pressure (IOP), which can cause damage to the optic nerve fibers. However, glaucoma can also occur with normal or even low IOP, highlighting the complexity and multifactorial nature of the disease. Given the aging global population, the prevalence of glaucoma is expected to rise, underscoring the importance of awareness, early diagnosis, and appropriate management strategies. Understanding the different forms of glaucoma, their pathophysiology, and the latest advancements in diagnostic and therapeutic approaches is essential for effective management and improving patient outcomes.
Glaucoma is not a single disease but a group of related conditions that affect the optic nerve and can lead to vision loss. The various types of glaucoma differ in their mechanisms, presentation, and treatment approaches. Understanding these differences is critical for accurate diagnosis and effective management.
Primary Open-Angle Glaucoma (POAG)
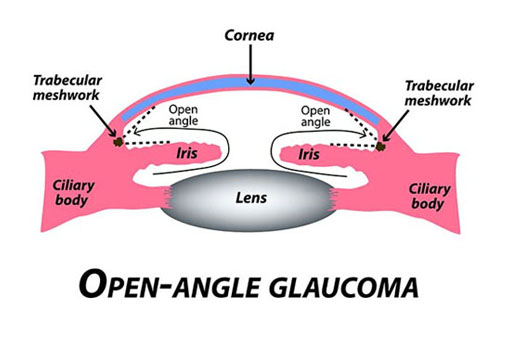
Primary Open-Angle Glaucoma (POAG) is the most common form of glaucoma, accounting for approximately 70-90% of all cases. It is characterized by a gradual increase in intraocular pressure (IOP) due to reduced drainage of aqueous humor through the trabecular meshwork. This slow progression often makes POAG asymptomatic until significant optic nerve damage has occurred.
Figure 1 (Mcmanes, 2020) |
Pathophysiology: The exact cause of POAG is not fully understood, but it is believed to involve dysfunction of the trabecular meshwork, leading to impaired aqueous outflow and elevated IOP. Over time, this increased pressure causes damage to the retinal ganglion cells and the optic nerve, resulting in peripheral vision loss that can progress to central vision impairment.
Risk Factors: Major risk factors for POAG include elevated IOP, advanced age, African or Hispanic ancestry, family history of glaucoma, and conditions such as diabetes and hypertension. Regular screening is recommended for individuals at higher risk to ensure early detection and intervention.
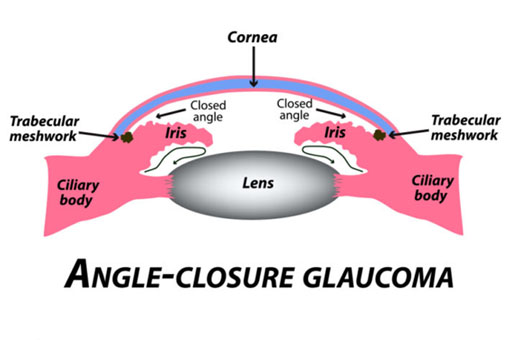
Angle-Closure Glaucoma
Figure 2 (Angle-Closure Glaucoma | Fort Lauderdale Eye Institute, 2018) |
Angle-Closure Glaucoma, also known as closed-angle glaucoma or narrow-angle glaucoma, occurs when the anterior chamber angle between the iris and cornea is too narrow, preventing adequate drainage of aqueous humor. This can lead to a sudden and dangerous increase in IOP.
Acute vs. Chronic: Angle-closure glaucoma can present in two forms:
- Acute Angle-Closure Glaucoma: This is an ophthalmic emergency characterized by a rapid rise in IOP, leading to severe eye pain, headache, nausea, and sudden vision loss. Immediate treatment is required to prevent permanent vision damage.
- Chronic Angle-Closure Glaucoma: In this form, the angle closure progresses more gradually, leading to a slower increase in IOP and a more subtle loss of vision. This can be more challenging to diagnose and may require different treatment approaches.
Normal-Tension Glaucoma
Normal-Tension Glaucoma (NTG) is a form of glaucoma where optic nerve damage occurs despite normal IOP levels. The pathogenesis of NTG is not fully understood, but it is thought to involve factors such as reduced blood flow to the optic nerve or increased susceptibility of the optic nerve to normal pressure.
Pathophysiology: The optic nerve in NTG patients may be more vulnerable to damage from normal IOP levels, possibly due to vascular insufficiency, genetic factors, or other unknown mechanisms. As with other forms of glaucoma, the disease leads to progressive vision loss, often without symptoms in the early stages.
Risk Factors: Risk factors for NTG include a family history of the condition, Japanese ethnicity, and systemic conditions such as low blood pressure or vasospastic disorders like migraine.
Secondary Glaucoma
Secondary Glaucoma results from another eye condition or systemic disease that increases IOP and damages the optic nerve. Common causes of secondary glaucoma include trauma, uveitis, neovascularization, pigment dispersion syndrome, and prolonged use of corticosteroids.
Causes:
- Trauma: Physical injury to the eye can damage the drainage angle, leading to secondary glaucoma.
- Uveitis: Inflammation of the uvea can lead to increased IOP due to the blockage of the trabecular meshwork by inflammatory cells.
- Steroid-Induced Glaucoma: Prolonged use of corticosteroids, whether topical, oral, or inhaled, can cause elevated IOP in susceptible individuals.
Congenital Glaucoma
Congenital Glaucoma, also known as pediatric glaucoma, is a rare form that occurs in infants and young children. It is often due to developmental anomalies in the eye’s drainage system.
Genetic Factors: Congenital glaucoma is frequently inherited, and mutations in specific genes, such as CYP1B1, have been associated with the condition. Early diagnosis and surgical intervention are critical to preventing vision loss in these young patients.
Early Signs and Detection: Symptoms in infants may include enlarged eyes (buphthalmos), tearing, light sensitivity, and corneal clouding. Regular pediatric eye exams are essential for early detection.
- Mechanism of Action: LipiFlow is a device designed specifically to treat Meibomian gland dysfunction (MGD), a major cause of evaporative dry eye. The device uses a combination of heat and gentle pressure to unclog and express the Meibomian glands, improving the quality of the lipid layer of the tear film and reducing symptoms of dry eye.
- Procedure: The LipiFlow device is applied to the eyelids, where it delivers controlled, therapeutic heat to the inner eyelid surface while simultaneously applying gentle pulsation to the outer eyelid. This process liquefies and removes blockages in the Meibomian glands, restoring their function.
- Indications and Efficacy: LipiFlow is indicated for patients with moderate to severe MGD who have not responded adequately to traditional therapies such as warm compresses and manual expression. Studies have shown that LipiFlow can significantly improve Meibomian gland function, increase tear break-up time, and provide lasting symptom relief for up to a year following treatment.
- Patient Selection: LipiFlow is particularly suitable for patients with chronic MGD and those seeking a more long-term solution to evaporative dry eye symptoms.
Glaucoma is primarily characterized by the progressive degeneration of the optic nerve, leading to vision loss. While elevated intraocular pressure (IOP) is a major risk factor, glaucoma can occur even in the presence of normal or low IOP, suggesting that other mechanisms are involved in the disease’s pathophysiology.
Optic Nerve Damage
The optic nerve, composed of over a million nerve fibers, is responsible for transmitting visual information from the retina to the brain. In glaucoma, these nerve fibers gradually deteriorate, leading to a characteristic pattern of optic neuropathy. The exact mechanisms underlying this damage are complex and multifactorial.
Role of Intraocular Pressure (IOP)
Elevated IOP is a well-established risk factor for glaucoma and is central to the disease process in most cases. IOP is determined by the balance between the production of aqueous humor by the ciliary body and its drainage through the trabecular meshwork and uveoscleral outflow. When this balance is disrupted, either due to increased production or decreased outflow of aqueous humor, IOP rises, exerting mechanical pressure on the optic nerve head.
Mechanical Theory of Optic Nerve Damage
According to the mechanical theory, elevated IOP leads to the deformation of the optic nerve head, specifically at the lamina cribrosa, where the nerve fibers exit the eye. This mechanical stress causes axonal damage, disrupting the transport of essential nutrients and signals to the optic nerve fibers, leading to their degeneration and eventual death.
Vascular Theory of Optic Nerve Damage
The vascular theory posits that optic nerve damage in glaucoma may also result from compromised blood flow to the optic nerve head. This ischemia can occur due to increased IOP compressing the microvasculature supplying the optic nerve or due to systemic vascular factors such as low blood pressure, vasospasm, or reduced ocular perfusion pressure. This insufficient blood supply leads to hypoxia and subsequent oxidative stress, contributing to the progressive loss of retinal ganglion cells.
Neurodegenerative Aspects
Recent research suggests that glaucoma shares some common features with neurodegenerative diseases such as Alzheimer’s disease. The involvement of excitotoxicity, where excessive glutamate release leads to neuronal damage, and mitochondrial dysfunction, which impairs cellular energy production, has been proposed in the pathophysiology of glaucoma. These insights have opened new avenues for potential neuroprotective therapies.
The clinical presentation of glaucoma varies depending on the type and stage of the disease. Early detection is crucial, as significant optic nerve damage and vision loss can occur before any noticeable symptoms arise.
Symptoms of Glaucoma
In many cases, especially with Primary Open-Angle Glaucoma (POAG), patients may be asymptomatic in the early stages. As the disease progresses, symptoms may include:
- Gradual loss of peripheral vision: Often unnoticed by the patient until the visual field is significantly constricted, leading to tunnel vision.
- Difficulty seeing in dim light: Patients may report challenges with night vision or adjusting to darkness.
- Blurred vision: This can occur intermittently, especially during spikes in IOP.
- Halos around lights: Particularly noted in angle-closure glaucoma.
- Eye pain, redness, and headaches: These are more common in acute angle-closure glaucoma, where IOP rises rapidly.
Diagnostic Techniques
Accurate and timely diagnosis of glaucoma relies on a combination of clinical evaluation, imaging, and functional tests.
- Tonometry: This test measures IOP, and is a critical component of glaucoma diagnosis. Techniques include Goldmann applanation tonometry, which is the gold standard, as well as non-contact tonometry.
- Ophthalmoscopy: Direct or indirect ophthalmoscopy is used to examine the optic nerve head for characteristic signs of glaucoma, such as cupping, notching, and thinning of the neuroretinal rim.
- Visual Field Testing: Perimetry tests, such as the Humphrey Visual Field test, assess the functional impact of glaucoma by mapping the patient’s visual field. It is essential for monitoring disease progression.
- Optical Coherence Tomography (OCT): OCT provides high-resolution cross-sectional images of the retina and optic nerve head, allowing for the precise measurement of retinal nerve fiber layer (RNFL) thickness and ganglion cell complex, which are crucial in early diagnosis and monitoring.
- Gonioscopy: This procedure allows for direct visualization of the anterior chamber angle, aiding in the differentiation of open-angle from angle-closure glaucoma and identifying secondary causes of increased IOP.
Role of Imaging in Glaucoma Diagnosis
Advances in imaging technology have significantly enhanced the ability to detect and monitor glaucoma. Baseline imaging, combined with serial follow-up assessments, helps in identifying disease progression and making informed treatment decisions. Technologies like enhanced depth imaging (EDI) OCT and confocal scanning laser ophthalmoscopy (CSLO) are now integral to the comprehensive evaluation of glaucoma patients.
The management of glaucoma aims to preserve vision by slowing or halting the progression of optic nerve damage. Treatment is tailored to the individual patient based on the type of glaucoma, severity, and response to therapy. The mainstay of treatment focuses on lowering IOP, as this is the only modifiable risk factor currently recognized.
Medical Management
Medical therapy is often the first line of treatment for most types of glaucoma, particularly POAG.
- Prostaglandin Analogs: These are the most commonly prescribed first-line drugs. They work by increasing uveoscleral outflow of aqueous humor, effectively lowering IOP. Examples include latanoprost, bimatoprost, and travoprost.
- Beta-Blockers: These reduce the production of aqueous humor. Timolol is a widely used beta-blocker, often in combination with other medications.
- Alpha Agonists: Medications like brimonidine decrease aqueous humor production and may also increase uveoscleral outflow.
- Carbonic Anhydrase Inhibitors: Available in topical and oral forms, these drugs reduce aqueous humor production. Dorzolamide and brinzolamide are common choices for topical application, while acetazolamide is used systemically in more acute settings.
- Rho Kinase Inhibitors: A newer class of medications that lower IOP by improving trabecular outflow. Netarsudil is an example.
Laser Therapy
Laser treatments offer an alternative or adjunct to medical therapy and can be particularly useful in patients who are non-compliant with medications or have contraindications to them.
- Trabeculoplasty: This procedure, including argon laser trabeculoplasty (ALT) and selective laser trabeculoplasty (SLT), improves aqueous humor drainage through the trabecular meshwork.
- Iridotomy: Performed in angle-closure glaucoma, this laser procedure creates a small hole in the peripheral iris to allow aqueous humor to flow more freely, preventing angle closure.
- Cyclophotocoagulation: This laser treatment targets the ciliary body to reduce the production of aqueous humor.
Surgical Options
When medical and laser treatments fail to adequately control IOP, or in cases of advanced glaucoma, surgical intervention may be necessary.
- Trabeculectomy: This is the most common surgical procedure for glaucoma, creating a new drainage pathway for aqueous humor to reduce IOP.
- Glaucoma Drainage Devices: Also known as shunts or tubes, these devices provide an alternative drainage route for aqueous humor. Examples include the Ahmed valve and Baerveldt implant.
- Minimally Invasive Glaucoma Surgeries (MIGS): These newer surgical techniques, such as the iStent or Trabectome, are designed to lower IOP with a lower risk of complications compared to traditional surgery. MIGS are often used in conjunction with cataract surgery.
Monitoring and Follow-Up
Regular follow-up is essential in the management of glaucoma, as it is a chronic, progressive condition. Monitoring typically includes periodic assessments of IOP, visual field testing, and imaging of the optic nerve and RNFL. Treatment plans should be adjusted based on disease progression and patient response.
Glaucoma research is rapidly evolving, with new insights and technologies continually emerging to improve diagnosis and treatment.
Gene Therapy and Neuroprotection
Gene therapy is a promising area of research, particularly for congenital forms of glaucoma. Techniques such as CRISPR-Cas9 are being explored to correct genetic mutations responsible for glaucoma. Additionally, neuroprotective strategies aim to preserve retinal ganglion cells and the optic nerve, potentially slowing disease progression regardless of IOP.
Advances in Diagnostic Technology
Artificial intelligence (AI) and machine learning are increasingly being integrated into glaucoma diagnostics. AI algorithms are being developed to analyze large datasets from visual field tests and OCT scans, providing more accurate and earlier detection of glaucoma progression. These tools also assist in risk stratification and personalized treatment planning.
Emerging Treatments and Future Directions
Research into novel pharmacological agents that target specific pathways involved in glaucoma is ongoing. For example, drugs that improve mitochondrial function or inhibit excitotoxicity may offer new avenues for treating glaucoma. Additionally, advances in drug delivery systems, such as sustained-release implants, are being explored to improve patient compliance and treatment efficacy.
Glaucoma remains a leading cause of blindness worldwide, but with advances in early detection and individualized treatment strategies, the prognosis for patients continues to improve. Ophthalmologists and eye care professionals must stay informed about the latest developments in glaucoma management to provide the best possible care. The future of glaucoma treatment holds promise, with ongoing research focused on neuroprotection, gene therapy, and innovative diagnostic tools, all aimed at preserving vision and enhancing the quality of life for those affected by this complex disease.
Reference:
Angle-Closure Glaucoma | Fort Lauderdale Eye Institute. (2018, August 24). https://flei.com/angle-closure-glaucoma/
Mcmanes, A. (2020, July 23). Primary open-angle glaucoma: Causes, risks and treatment. All about Vision. https://www.allaboutvision.com/conditions/primary-open-angle-glaucoma/